- Mercury's Newsletter
- Posts
- Mercury Newsletter October 2025
Mercury Newsletter October 2025
Mercury, simplifying life science shipping

Welcome
Thanks for checking out Mercury’s October Newsletter! This issue brings a mix of helpful industry shipping tips, time-saving product updates, and an employee spotlight of someone we’re proud to have on the team working for you! Let us know if there’s anything you’d like us to cover on our next quarterly newsletter.
 | Upcoming EventDiagnostics Shipping WebinarDate: December 4th, 2025 Registration: Registration Link Growth without the budget blow-up: we’ll share the playbook for scaling kits and specimens while keeping service high; packaging choices, lane design (hybrid parcel vs. NFO), and compliant workflows that actually save. |
New Mercury Video
We’re excited to share Mercury’s new explainer video! A huge thank you to everyone who helped bring it to life, it’s a great showcase of how Mercury supports our clients. We also captured some amazing customer testimonials now live on our YouTube channel. Thank you especially to our clients who shared their stories, it was a pleasure hearing how much you value working with your Mercury squads!
Industry Knowledge

Tariff Shifts: What They Mean for Pharma Logistics
As tariffs escalate, businesses face decisions on whether to absorb costs, adjust pricing strategies, or explore reshoring options to reduce exposure. Such changes can disrupt established supply routes, delay product availability, and affect patient access. Understanding these dynamics is critical for preparing resilient, compliant, and cost-effective logistics strategies in an evolving trade environment.

Streamline Your CRO/CMO/CDMO Inbound Logistics
Inbound shipments from research and manufacturing partners are critical to keeping clinical trials and pharmaceutical production on schedule. When shipments are fragmented or tracked manually, organizations risk compliance issues and missed timelines. By standardizing processes, consolidating data, and ensuring real-time tracking, companies can strengthen control over inbound logistics and maintain consistency across suppliers. This approach safeguards sensitive products and supports faster, more reliable delivery.
Ensuring Safe and Compliant Biological Shipments
Shipping biological substances requires precise classification and strict compliance with international regulations. Missteps can result in fines, shipment delays, or safety risks that disrupt critical healthcare and research operations. Each category whether Category A, Category B, genetically modified organisms, or exempt specimens carries its own requirements for labeling, packaging, and documentation. By understanding these standards and applying them consistently, organizations protect their supply chains, maintain compliance, and ensure that vital specimens reach their destination without interruption.

Validating Packaging for Gene Therapy Shipments
Gene therapies are among the most delicate treatments in modern medicine, requiring ultra-cold conditions and rigorous controls throughout transport. Stress testing, transit simulations, and qualification protocols are essential steps to confirm reliability. Further, without proper packaging validation, even minor temperature fluctuations or handling errors can compromise the integrity of these advanced therapies. By implementing validated packaging systems and maintaining audit-ready records, organizations can meet regulatory expectations while ensuring life-saving treatments reach patients safely.
Product Spotlight: Accounts
Accounts and Sub-Accounts for better billing Sites have been replaced by Accounts, with the option for Sub-Accounts. Sub-Accounts allow you to track billing in an intuitive way as you grow or expand your shipping use-cases. When you create any new account, you can associate it with an address which will then also be added to your company’s Address Book. | 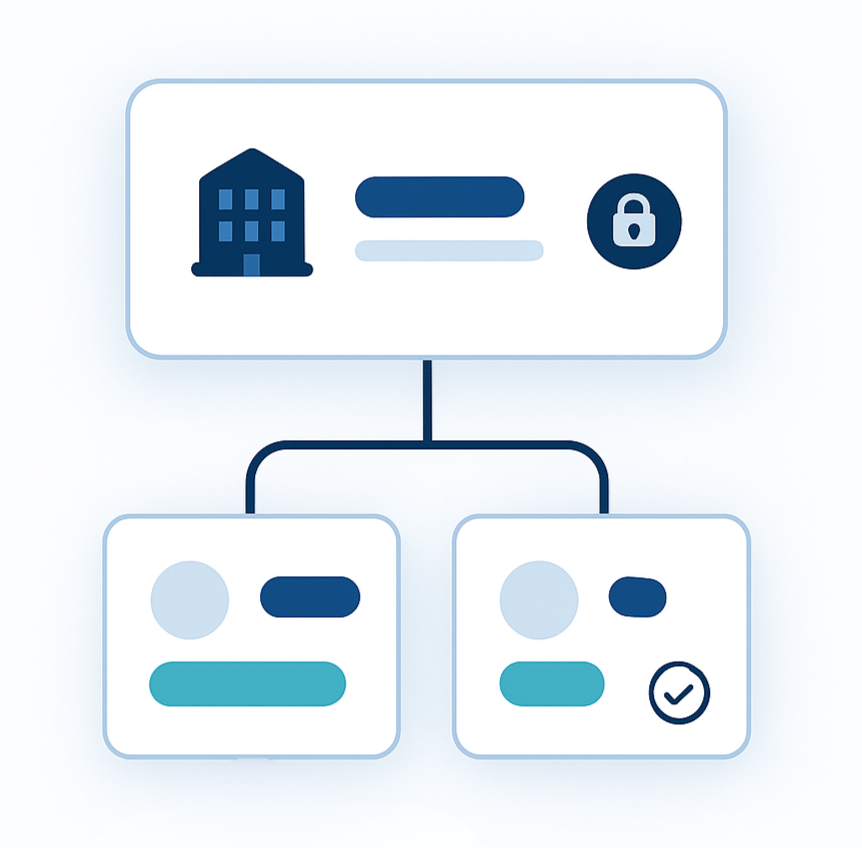 |
Additional Software Updates:
Download commercial invoices instantly
A new Quick Action on the Tracking page lets you download the commercial invoice for international parcel and cold-chain shipments whenever it’s available as an e-document (not when it’s embedded in the label PDF).
Cancel FedEx pickups in the Portal
You can now cancel scheduled FedEx pickups directly from the Pickup page, no call or chat needed.
All shipment documents in one place
Public shipment documents are now easy to access via the Portal. You’ll see them listed with name, type, and date.
Recent sender addresses
Your top five most-used sender addresses appear now appear first in the Origin dropdown. When you pick one, we’ll keep the logged-in user as the sender contact (phone + email), rather than the saved contact on that address.
Book Same-day in the Portal
You can now create intra-country same-day shipments right in the Portal. For same-day shipments across international borders continue to call or email the Mercury team.
Account deactivation (Admins)
Admins can request account deactivation in the Portal. Once submitted, the account will no longer appear as a billing option for your team.
Employee Spotlight

Dalton Puller - Senior Logistics Guide
At Mercury, every successful delivery reflects the dedication of our team. Dalton Puller, a Logistics Guide in our operations team, embodies that commitment.
With years of logistics experience, Dalton approaches each challenge with calm focus, a skill honed from both his career and his unique background, including his time as a semi-professional racecar driver. Whether resolving customs delays in Italy or coordinating early-morning courier pickups, he relies on organization, teamwork, and trusted partnerships to deliver critical results for our clients.
Mercury is thankful to have a problem-solver like Dalton on our team! Plus, we enjoy his racecar stories, though with our proper planning we should never need to put his speedy-driving skills to the test!